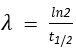Home » EasyPhys » From the Universe to the Atom » Properties of the Nucleus » Examine the model of half-life in radioactive decay and make quantitative predictions about the activity or amount of a radioactive sample
Examine the model of half-life in radioactive decay and make quantitative predictions about the activity or amount of a radioactive sample
The rate of decay of an radioactive isotope is measured by its half life.
Half life : It is the time required for radioactive isotope to reduce to half of its initial value.
- It has been found that the rate at which radioactive isotopes decay , is directly proportional to the number of atoms present. The rate can be expressed as :

- (where
 is the decay constant , with units s-1 )
is the decay constant , with units s-1 )
- This equation can be integrated to give the following result :

- where,
- No is the number of atoms present at time t = 0
- Nt is the number of atoms present at time t
- Using the definition of half life , we can find

 is when the number of atoms are half the original amount, i.e.
is when the number of atoms are half the original amount, i.e.
- Thus ,

- rearranging we get ,

- Thus if we can measure the half life, we can calculate the decay constant.
Extract from Physics Stage 6 Syllabus © 2017 NSW Education Standards Authority (NESA)