Home » EasyPhys » From the Universe to the Atom » Properties of the Nucleus » Model and explain the process of nuclear fission, including the concepts of controlled and uncontrolled chain reactions, and account for the release of energy in the process
Model and explain the process of nuclear fission, including the concepts of controlled and uncontrolled chain reactions, and account for the release of energy in the process
Nuclear fission is a nuclear reaction or a radioactive decay process, in which the nucleus of an atom splits into smaller/lighter nuclei.
Artificially induced nuclear reaction :
- A heavy atom such as an isotope of Uranium or Plutonium, is bombarded with a subatomic particle to create an unstable nucleus such that it breaks into two lighter elements.
- The difference in binding energy of the original constituents and the resulting products is released as energy.

An example of a very common Nuclear fission reaction :
U235 is bombarded with neutrons , which creates the products Krypton , Barium and more neutrons. The fission of one atom of U235 releases 202.5MeV of energy . The energy comes from the difference in binding energy between that of the Uranium and Krypton-Barium.
![]()
- If any of the 3 neutrons (from such reactions as shown above), strikes another nucleus and causes another nuclear fission reaction , then a chain of fission reactions can continue.
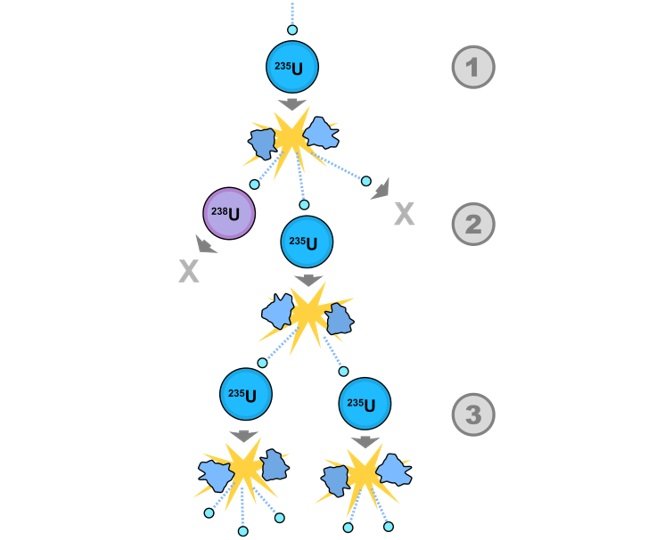
- Fissile material : If any material is capable of sustaining a chain reaction, it is called fissile material.
- Critical mass : for the chain reaction to continue, a minimum amount of fissile material is required. This amount is called critical mass.
- Chain reactions can be controlled and uncontrolled.
Controlled chain reaction:
The chain reaction depends on the free neutrons striking the Uranium atom. Thus by controlling the number of neutrons allowed to strike the Uranium atom, chain reactions can be controlled.
Control rods are used to absorb the excess neutrons . These rods are generally made of Boron or Cadmium.
Uncontrolled chain reactions:
When the number of neutrons cannot be controlled , the chain reaction grows at every step. These leads to an uncontrolled chain reaction which may lead to an explosion.
Extract from Physics Stage 6 Syllabus © 2017 NSW Education Standards Authority (NESA)
