Home » EasyPhys » From the Universe to the Atom » Structure of the Atom » Investigate, assess and model the experimental evidence supporting the existence and properties of the electron
Investigate, assess and model the experimental evidence supporting the existence and properties of the electron
- Cathode Rays: Streams of electrons emitted within an evacuated tube from a cathode to an anode.
- Cathode Ray Tube: Also known as a discharge tube, a highly evacuated sealed-glass tube with two electrodes contained within it, through which cathode rays can travel.
- Cathode rays were discovered in 1855 by German physicists Heinrich Geissler and Julius Plucker.
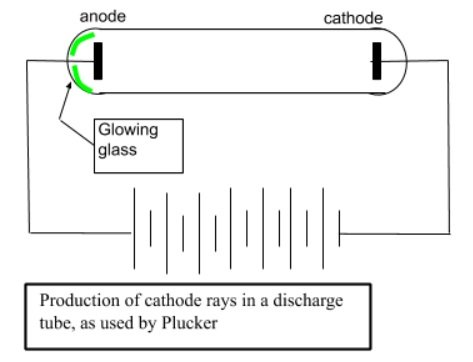
- In 1875, William Crookes designed a number of different discharge tubes to study cathode rays.
- There were two main groups of scientists who supported two different theories about cathode rays:
- German physicists, such as Hertz and Lenard, who advocated an electromagnetic wave model.
- English physicists, such as Crookes and Thomson, who advocated a charged particle model
- In 1897, J. J. Thomson demonstrated that cathode rays were actually deflected by electric fields, which led to the acceptance of the charged particle model.
Thomson’s charge-to-mass experiment
- To find the charge/mass ratio of cathode rays (now known as electrons), J. J. Thomson performed two main steps:
- Varying perpendicular magnetic and electric fields acting on a beam of cathode rays until the beam was not deflected, and then equating electric and magnetic force equations, determining the velocity of the cathode rays.
- Applying the same strength magnetic field alone and determining the radius of the circle path travelled by the particles.
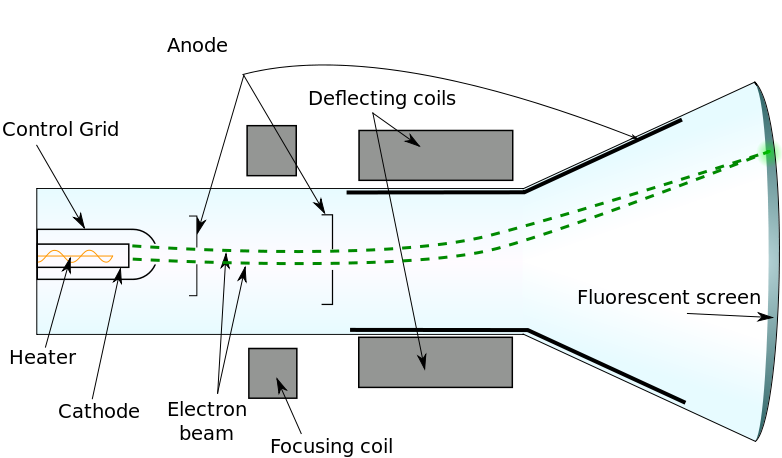
- More specifically:
- He directed a beam of cathode rays through perpendicular electric and magnetic fields.
- He adjusted the strength of the electric field until the beam passed through the perpendicular fields without being deflected.
- For this to occur, the forces exerted by the two field would be equal, which allowed the formation of a mathematical expression:
- Fb = FE
- qvB = qE
- Hence ,

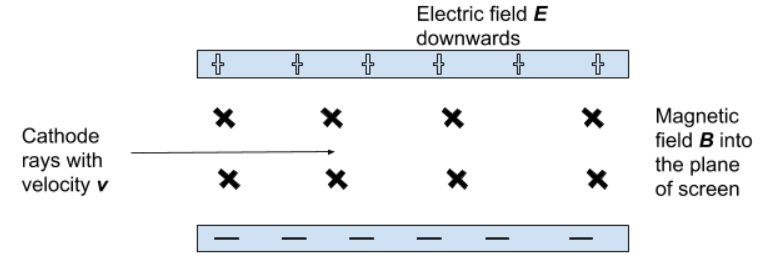
- Thus, the velocity of the cathode rays could be determined.
- Then, the beam was passed through the same magnetic field alone and the radius of the curvature of the beam was measured.
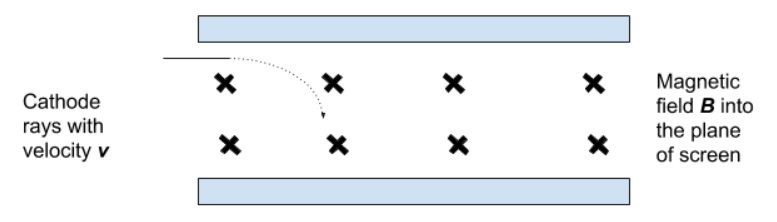
- Because the magnetic field exerts a centripetal force:
- FC = FB

- Hence,

- Thus, the charge/mass ratio could be determined.
- Because the magnetic field exerts a centripetal force:
- Thomson confirmed the value found by passing the beam through the same electric field alone and measuring the radius of the curvature of the beam.
- Thomson determined the charge/mass ratio to be 1.759 x 1011 C kg-1
- As electrons produced by different cathodes had identical properties, Thomson concluded that electrons were a subatomic building block of all atoms.
Millikan’s oil drop experiment (ACSPH026)
- In 1909 , Robert Millikan studied fine drops of oil within a uniform electric field to understand charge of a particle.
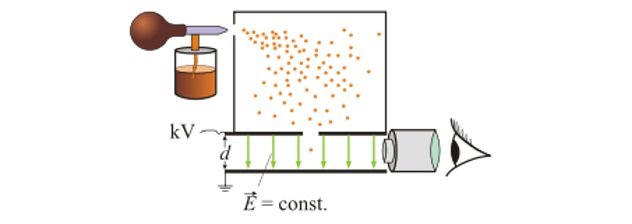
- His setup is shown as above.
- When the oil drops were sprayed , some of them drifted down to the region of uniform electric field.
- As the oil drops entered the electric field, they were also momentarily exposed to X-rays so that the oil drops would get charged.
- Once charged the electric field would act on the oil drops.
- Two forces would be acting on the charged oil drop in this region:
- Gravitational force :

- Electric Force :

- Gravitational force :
- The electric field could be adjusted such that , and the oil drops move with constant velocity or are stationary.
- this implies :
- This motion of oil drop could be observed and verified with the help of a microscope.
- this implies :
- To measure the mass of the oil drop, Millikan did the following:
- allowed the oil drop to fall without electric field.
- measured the terminal velocity
- using equations of fluid mechanics, calculated radius and hence volume of the oil drop.
- Using an oil of known density, and measured volume, calculated the mass of the drop.
Millikan observed that the charge appeared in quantised values. It was in multiple of a particular value.
He found the value to be 1.6 x 10-19 C, now understood as the charge of the electron.
He received a Nobel prize for this discovery in 1923.
Extract from Physics Stage 6 Syllabus © 2017 NSW Education Standards Authority (NESA)
