Home » EasyPhys » The Nature of Light » Light: Quantum Model » Investigate the evidence from photoelectric effect investigations that demonstrated inconsistency with the wave model for light
Investigate the evidence from photoelectric effect investigations that demonstrated inconsistency with the wave model for light
The photoelectric effect refers to the emission, or ejection, of electrons from the surface of, generally, a metal , when it is exposed to incident electromagnetic radiation.
- The Photoelectric effect was first observed by Hertz in 1887 when investigating the production and detection of electromagnetic waves using a spark gap in an electric.
- Hertz did not understand why ultraviolet radiation had an effect on the spark length, and he did not pursue the discovery.
- The ultraviolet radiation increased the maximum spark length by freeing electrons in the metal.
- In 1899, J. J. Thomson established that the ultraviolet light caused electrons to be emitted from a sheet of zinc metal and showed that these electrons were the same particles found in cathode rays.
- In 1902, German physicist Philipp von Lenard performed experiments that demonstrated:
- The number of electrons emitted is proportional to the light intensity.
- The kinetic energy of emitted electrons is not related to the light intensity.
- The energy of emitted electrons is related to the light frequency.
- There is a threshold frequency that must be reached before electrons are emitted.
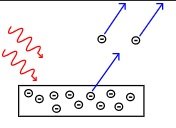
Classical physics predicted that energy from radiation could be absorbed slowly over time, until electrons had gained sufficient energy to escape the surface. Thus these observations were unable to be explained by classical physics.
Extract from Physics Stage 6 Syllabus © 2017 NSW Education Standards Authority (NESA)
